Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
A groundbreaking study by researchers at Johns Hopkins University suggests that a simple blood test could detect cancer years before symptoms manifest. This could revolutionize early diagnosis and prevention, significantly improving survival rates.
Early detection remains one of the most significant challenges in cancer treatment. Identifying the disease in its early stages often leads to more effective treatment options and better patient outcomes.
Early Detection: A Critical Advantage
The study, published in Cancer Discovery, highlights the importance of early intervention. According to researcher Yuxuan Wang, detecting cancer "three years earlier provides time for intervention," when tumors are likely less advanced and more curable. This time advantage could be the difference between life and death, particularly for aggressive cancers.
The Science Behind the Breakthrough
The research focuses on circulating tumor DNA (ctDNA), genetic material that tumors shed into the bloodstream. While these traces are minute, especially in the early stages, scientists have developed sophisticated algorithms to identify modifications in DNA patterns linked to tumors. This technique forms the basis of a Multi-Cancer Early Detection (MCED) test, designed to detect cancer-specific genetic changes in the blood.
Study Results: Promising but Not Perfect
The research team analyzed blood samples from 52 individuals, divided into two groups:
- 26 people diagnosed with cancer within six months of sample collection.
- 26 people who remained cancer-free.
The MCED test flagged eight cancer cases, representing a 31% detection rate. Importantly, this detection occurred before formal diagnosis or visible symptoms, showcasing the potential for early identification.
Detecting Cancer Years in Advance
Further analysis of older blood samples from participants revealed even more promising results. Cancer signals were found in four out of six samples taken 3.1 to 3.5 years before diagnosis. Although the ctDNA levels were lower than the current test threshold, the findings suggest that tumors begin shedding DNA into the blood long before symptoms arise.
The Path Forward
While these results are encouraging, they also underscore the need to improve the sensitivity of current technology. Early-stage cancers have lower ctDNA levels, making detection challenging.
Dr. Bert Vogelstein, a senior cancer researcher, emphasizes the promise of MCED tests but also stresses the need for higher sensitivity.
What Happens After a Positive Blood Test?
Moving from the lab to clinical practice requires rigorous clinical trials to prove the reliability and safety of blood-based cancer screening tests. Regulatory approvals are also necessary before widespread adoption.
Dr. Nickolas Papadopoulos from the Ludwig Centre raises the crucial question of clinical follow-up after a positive test, including further scans, biopsies, and potential preventive treatments.

A Hopeful Future
Despite current limitations, this research signifies a positive step in cancer diagnostics. Coupled with ongoing advancements in cancer treatment, particularly therapies targeting multiple cancer types, the future offers the potential for dramatically improved survival rates. This breakthrough could revolutionize cancer screening and treatment, providing hope for earlier detection and more effective interventions.
Newer articles
Older articles
-
 Aamir Khan’s Sitaare Zameen Par picks up momentum on release day as last-hour bookings surge past 10,000 tickets
Aamir Khan’s Sitaare Zameen Par picks up momentum on release day as last-hour bookings surge past 10,000 tickets
-
 Are we living inside a black hole? NASA’s James Webb findings stun scientists
Are we living inside a black hole? NASA’s James Webb findings stun scientists
-
 Vivek Oberoi reveals how shifting to Dubai helped him build a Rs 1200 crore real estate empire: ‘This feels more like home now’
Vivek Oberoi reveals how shifting to Dubai helped him build a Rs 1200 crore real estate empire: ‘This feels more like home now’
-
 'Would be remarkable to choose someone else if their last knock was a 170'
'Would be remarkable to choose someone else if their last knock was a 170'
-
 Jaya Bachchan believes Amitabh Bachchan “gave a better performance” than her in ‘Mili’ - Exclusive
Jaya Bachchan believes Amitabh Bachchan “gave a better performance” than her in ‘Mili’ - Exclusive
-
 Amitabh Bachchan calls working with son Abhishek Bachchan his 'greatest blessing'
Amitabh Bachchan calls working with son Abhishek Bachchan his 'greatest blessing'
-
 Sunjay Kapur’s sister Mandhira shares rare selfie after his funeral: 'We are incomplete without you'
Sunjay Kapur’s sister Mandhira shares rare selfie after his funeral: 'We are incomplete without you'
-
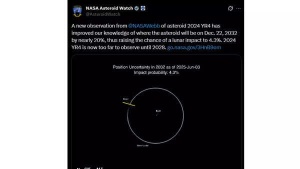 ‘City killer’ asteroid may hit the Moon in December 2032 could threaten satellites around Earth; experts warn
‘City killer’ asteroid may hit the Moon in December 2032 could threaten satellites around Earth; experts warn
-
 Peel or without peel: Which is the best way to have almonds for maximum nutrition
Peel or without peel: Which is the best way to have almonds for maximum nutrition
-
 Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals